Vial of Moderna COVID-19 Vaccine:
Moderna Inc’s COVID-19 antibody on Friday turns into the second to get crisis use authorization from the United States Food and Drug Administration (FDA). Welcome news to a country with a stunning Covid-19 loss of life of more than 307,000 lives lost[1].
A large number of portions of the Moderna antibody rely upon to add to the US rollout. It starts for the current week with medical services laborers. More seasoned individuals in long haul care offices are next in line for immunizations. With a US Centers for Disease Control and Prevention master board on Sunday set to suggest what gatherings follow. As enterprises contend to have their laborers given priority[1].
FDA Authorization:
The FDA declared the authorization the day after the organization’s board of outside specialists support its utilization. Seven days after the FDA approves an immunization from Pfizer Inc and German accomplice BioNTech SE[1].
Immunization from Pfizer:
The immunization from Pfizer and BioNTech in light of comparative innovation. It has place into the arms of thousands of US medical care laborers this week in a huge cross-country rollout. Moderna infusions require to start in the coming days for grown-ups 18 years of age and up[1].
“With the accessibility of two immunizations now for the avoidance of Covid-19. The FDA has made another pivotal stride in the battle against this worldwide pandemic that causing tremendous quantities of hospitalizations in the United States every day”. FDA Commissioner Stephen Hahn, MD, said in an explanation[1].
How to Arrive Vaccine at Clinics:
Moderna’s shot requires to utilized in harder-to-arrive areas, for example, country medical clinics. The immunization should put away and send frozen, yet doesn’t need the super cool temperatures of the Pfizer/BioNTech shot[1].
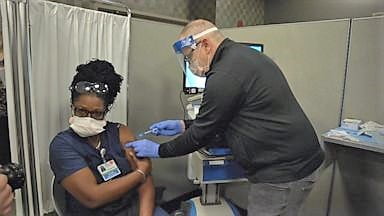
How Antibody Improvement in Body Against COVID-19:
The speed of antibody improvement, not exactly a year from when the principal instance of the new COVID-19 found in the United States. It is a staggering logical achievement, in spite of the fact that there is some aversion among the general population[1].
“My expectation that all Americans will secure themselves by getting inoculate when the immunization opens up to them. That the means by which our nation will start to mend and push ahead”. The top US irresistible illness researcher Anthony Fauci said in an explanation. Moderna said it expected to apply for a full US permit in 2021[1].
FDA Principal Administration:
The FDA choice denotes the principal administrative authorization on the planet for Moderna’s immunization. Its approves of its courier RNA innovation demonstrated to be almost 95 percent viable with no genuine security concerns. It comes not exactly a year after the main Covid-19 case was distinguished in the United States[1].
The antibody, created in association with the National Institutes of Health. It had generally minor results including torment around the infusion site and growing[1].
A biotech organization with the US Government:
The biotech organization has worked with the US government. So, the government to get ready for the dispersion of 5.9 million shots beginning as right on time. When defrosted, the Moderna antibody can keep at common cooler temperatures. It is directed in two shots 28 days separated[1].
Between the two immunizations, the United States is expecting 40 million dosages before year-end. It is enough to in the end inoculate 20 million individuals, as both require two shots. “More brilliant days lie ahead,” reacted US President-elect Joe Biden, who intends to be inoculated on Monday[1].
Donald Trump with the Authorizations:
US President Donald Trump on Twitter speaks to the authorization. “Congrats, the Moderna immunization is presently accessible!” he composed. The antibody should be shipped to emergency clinics and different focuses before infusions start[1].
Moderna said it would convey roughly 20 million dosages to the US government this year and expected to have between 100 million and 125 million around the world. The principal quarter of one year from now, with 85-100 million of those for the United States[1].
Moderna has managed the US government to give an aggregate of 200 million portions before the finish of June 2021. Different antibodies actually are in trying, including a one-shot infusion from Johnson and Johnson. The two-shot course from AstraZeneca and Oxford University[1].

Deaths in The United States of America:
US hospitalizations and deaths have flooded, driven by a month ago’s Thanksgiving occasion social affair. Specialists have recharged limitations and closures the nation over. General wellbeing authorities have cautioned diseases from Christmas and New Year’s festivals could compound an emergency that as of now takes steps to overpower medical care frameworks cross country[1].
Antibodies Successfully Worked for Public:
Certainly, even with two exceptionally successful antibodies, practices, for example, social separating and face-covering will be required for quite a long time before enough individuals are vaccinated to control infection transmission and at last end the pandemic[1].
“With solid government financing for quick immunization rollout and schooling, and by following the direction of general wellbeing specialists to keep wearing veils and looking after distance, we can bring the finish of this pandemic closer,” Barbara Alexander, top of the Infectious Diseases Society of America, said in an explanation[1].
Conclusion:
The United States of America made the Moderna COVID-19 vaccine which the elderly uses for curing the public. Donald Trump addressed the authorities about the COVID-19 vaccine. The vaccine should be available in clinics as soon as possible.
References:
1. dawn. 19th December 2020; Available from: https://www.dawn.com/news/1596648/us-authorises-moderna-covid-19-vaccine-elderly-next-in-line-for-shots.